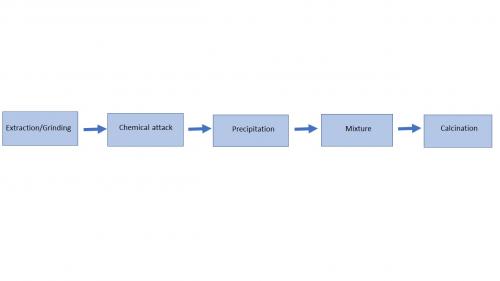
Outside of natural materials, the majority of powders used for advanced ceramics are produced by chemical synthesis in order to obtain pure phases instead of a mixture of compounds. There are numerous variations of the process, but they generally all adopt the same principles of chemical attack of the mineral ore, then precipitation/solidification and lastly calcination. The most well-known process is the Bayer process which is used to produce alumina of relatively high purity.
General principle
The natural raw materials are extracted and finely ground. They are then subjected to chemical attack to dissolve them in water (for the Bayer process, the bauxite is attacked with soda, for example). Then, by changing the temperature or adding additives, only the material required is precipitated, without the other impurities present in the ore. The chemicals obtained can be purified using various methods (solubilisation/precipitation, separation by sifting, distillation, etc.), and mixed with other salts to obtain the desired chemical composition (doping with MgO for alumina, and with Y2O3 for zirconia…). Lastly, this powder is calcinated at high temperature to obtain the desired crystalline phase.
Materials involved
Most oxide powders are synthesised in this way. In particular, the materials synthesised by members of the Association are alumina, zirconia, spinel and alumina/zirconia composites.